Products
Pipeline
| Discovery | Pre-Clinical Data | Phase I | Phase II | FDA Approval | Market Entry | |
|---|---|---|---|---|---|---|
| Gastrointestinal Products | ||||||
| GI-Gel™ |
|
|||||
| EsophaGel™ |
|
|||||
| MatriSeal™ |
|
|||||
| MucosaLift™ |
|
|||||
| Hemostatic Product | ||||||
| MatriStat™ |
|
|||||
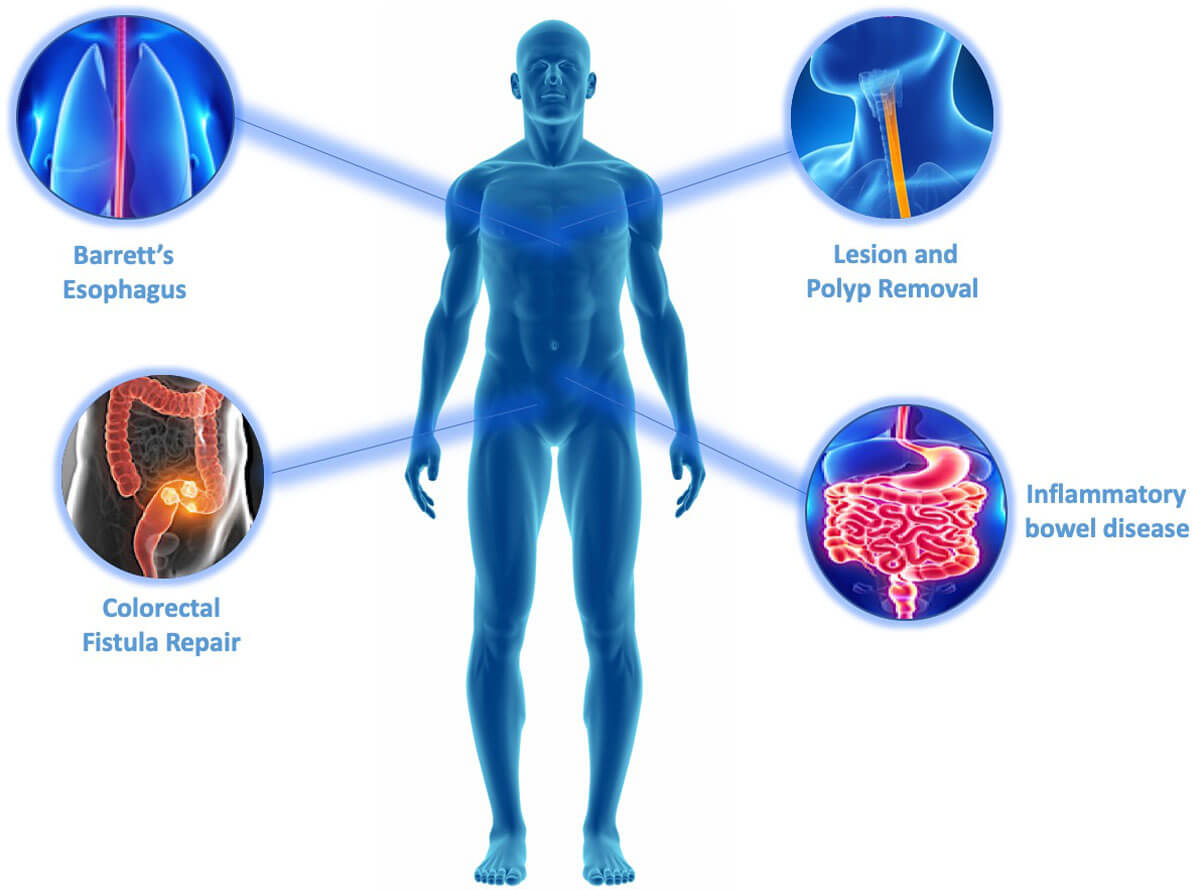
GI Product Pipeline*
Download PDF* Products listed above are in various stages of development and are not currently FDA approved or available for commercial use.